75%
Site burden reduction (4)
60%
Cost savings in imaging collection and storage (1)
90%
Cost savings in advanced image analysis (2)
80%
Time savings by automated advanced image analysis (up to 98%) (3)
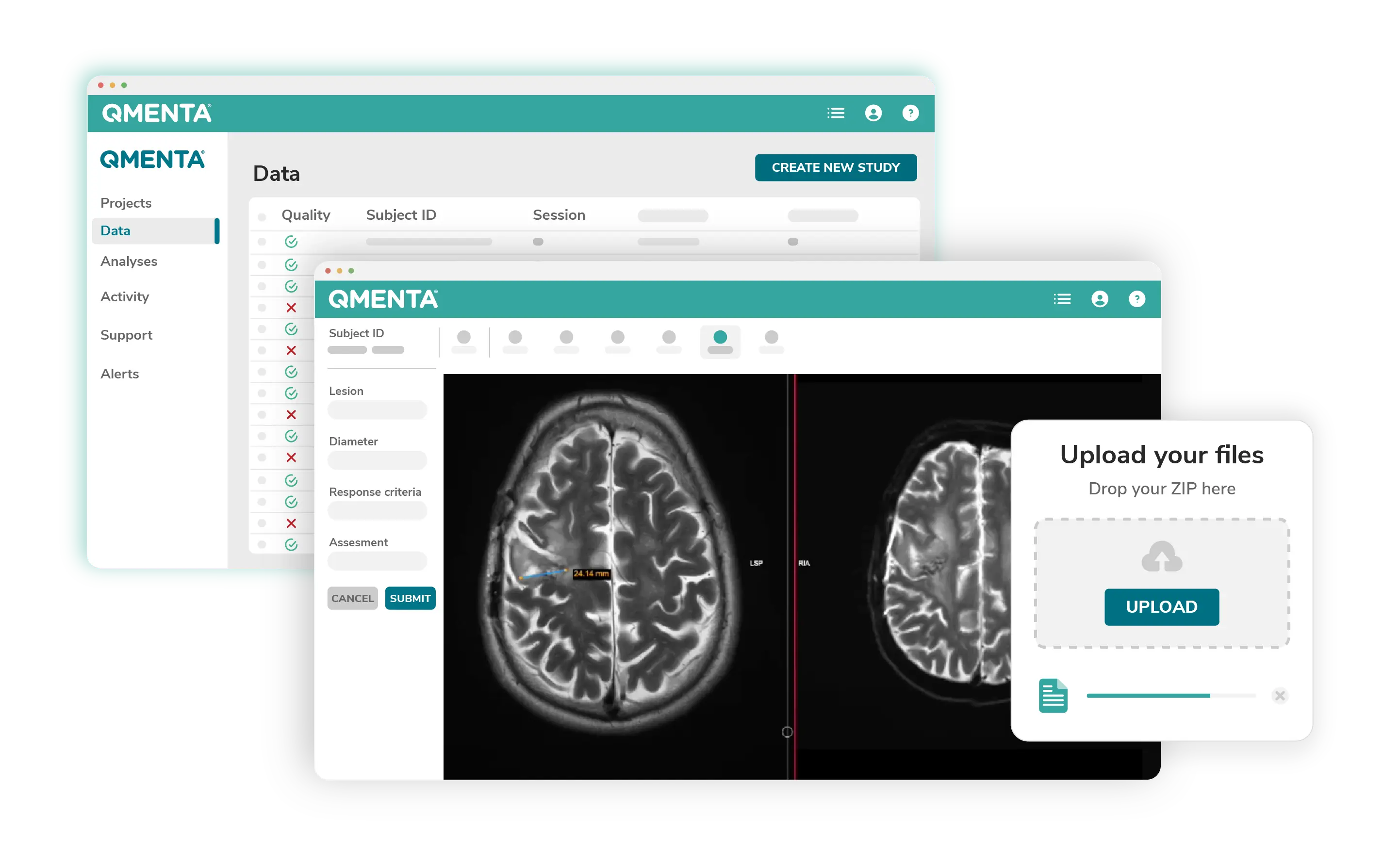
Everything You Need to Boost Productivity and Take Faster and More Accurate Decisions
Delivering Medical Imaging Outcomes With High Quality, Reliability, and Accuracy
All-in-one, secure and inspection-read Imaging Hub
- Intelligent data solutions including automatic de-identification, protocol adherence and QA checks
- Optimized for imaging, flexible for all modalities and data types with simple integration
- Trusted and secure, meeting the highest standards of compliance requirements
Enhanced Central Radiology Reading Solutions
- Zero footprint reading with FDA compliant viewer, enhanced by AI biomarker analysis, and Auto filling forms
- Configurable workflows, adjudication reading, assignability with audit trails
- Expert reading service, either your own or our network of expert radiologists
Expert Support by our Highly Credentialed Team
- We provide consultancy and services for imaging study design and site setup, ensuring tailored planning and seamless implementation
- Disease biomarker and analysis advisory and execution support
- Full study duration support for technical, scientific and project management needs
Imaging Areas of Expertise
CNS
QMENTA was founded by a team of expert neuroscientists, thus anchoring neuroscience as our core area of expertise and proficiency.
Oncology
Our collaborations with renowned oncology institutions have allowed us to develop sophisticated AI tools for oncology, such as our tumor segmentation and RANO assessment solutions.
Medical Devices
Leveraging our deep imaging expertise, we have played a pivotal role in the development of medical devices, integrating innovative imaging technologies to empower research teams.
AI software devices
Leveraging our deep expertise in AI imaging biomarkers, our platform offers an ideal ecosystem for scaling the use and validation of these innovative algorithms.
Rare Diseases
Leveraging AI, QMENTA transforms complex data from rare diseases like ALD and Rett Syndrome into actionable insights for research.
Other Therapeutic Areas
Our work extends beyond core specialties, encompassing various imaging research-related areas. Our diverse portfolio reflects our ability to innovate and adapt to a wide range of research challenges.
We are the main imaging platform of top CROs, pharmaceutical, and medical devices companies, take a look at some of our case studies
 Streamlining Medical Imaging to Support Alzheimer’s Disease Phase II Trial
Streamlining Medical Imaging to Support Alzheimer’s Disease Phase II Trial
 Cleveland Clinic Aims to Improve Multiple Sclerosis Diagnosis By Studying New Central Vein Sign Biomarker
Cleveland Clinic Aims to Improve Multiple Sclerosis Diagnosis By Studying New Central Vein Sign Biomarker
 Co-development of Digital RANO Tumor Segmentation Tool & Execution of the Brain Matrix Consortium Study
Co-development of Digital RANO Tumor Segmentation Tool & Execution of the Brain Matrix Consortium Study
Interested to know more?
Get in touch with our team for a 15-min demo or get your personalized RFP
- QMENTA calculation compared to cost of local computational resources
- Comparing the average hourly cost of radiologists vs. QMENTA’s computational cost
- Comparing hours spent by radiologist vs. tool compute time
- As reported by Amylyx Pharmaceuticals, CSO